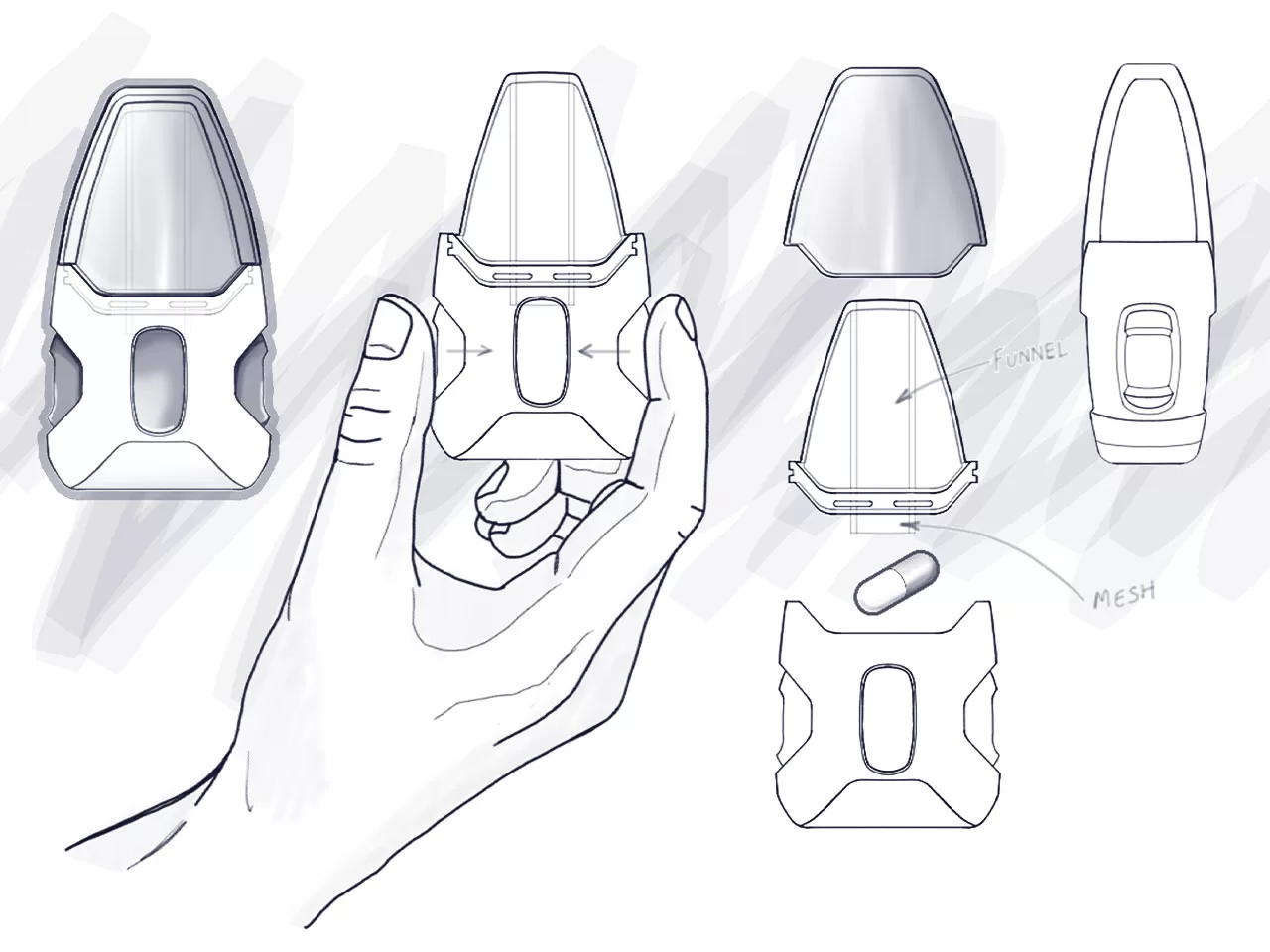
INDUSTRIAL DESIGN
Industrial design is so vital that it can determine the success or failure of a medical device. Effective and intuitive industrial design is the link that connects your technology with the people who need and use it most.
At Neucin, we are passionate about creating meaningful and trailblazing medical device experiences that are feasible and flexible to the challenging and changing demands of users and the market. Our goal is always to optimise performance, usability, and marketability to achieve our client’s vision.
Ultimately our carefully considered human-centred approach to industrial design drives our intentional aesthetics and innovative results.
- Concept ideation, sketching, design, development and testing.
- Model making and rapid prototyping and production of block models for early functional, ergonomic and aesthetic assessment.
- Product rendering, visualisation and animation.
- Technology scoping, technical feasibility and intellectual property assessment.
- Product visual brand and language development.
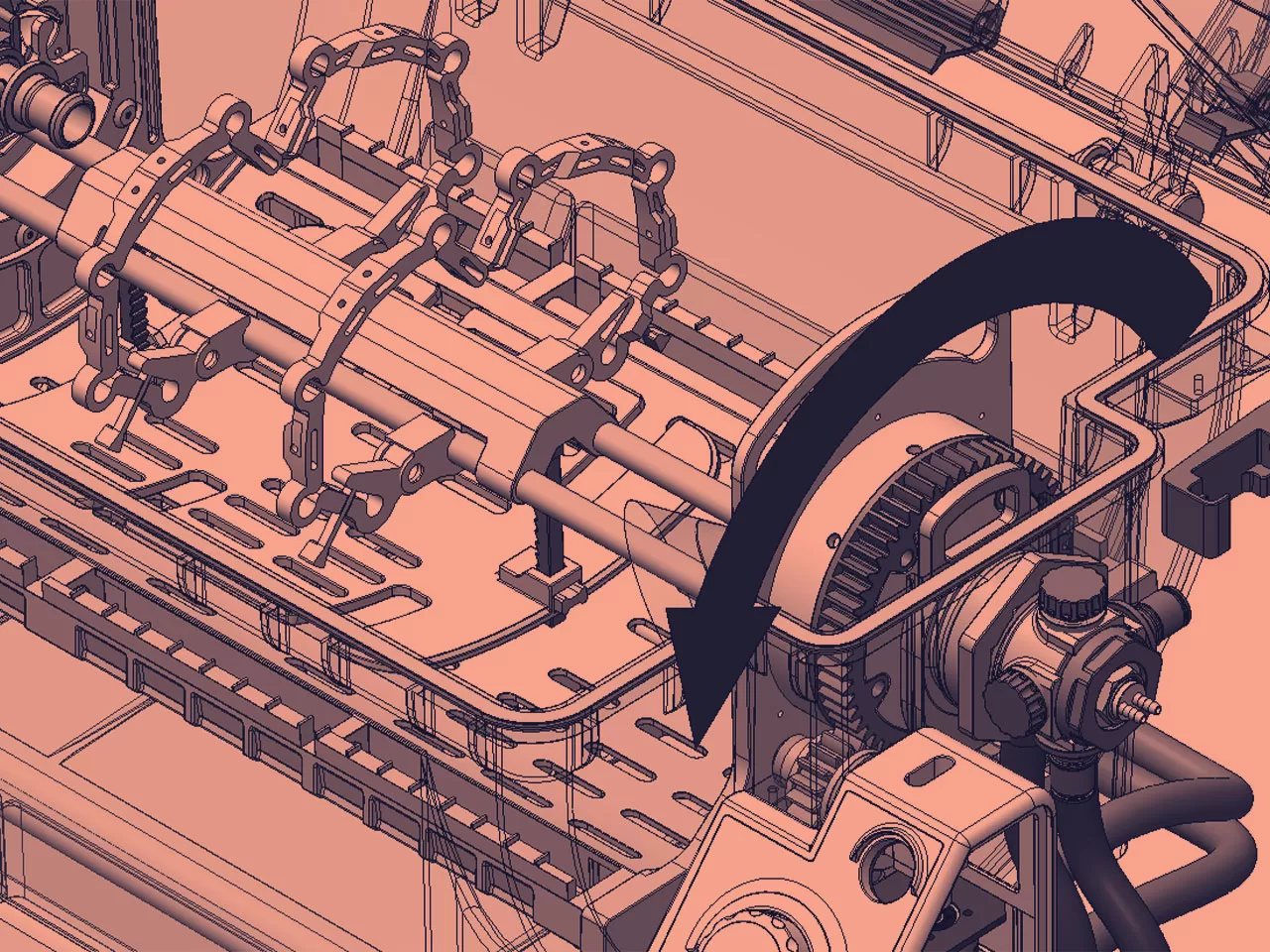
MECHANICAL ENGINEERING
As part of our mechanical engineering tasks, we undertake mathematical modelling, system characterisation, FEA, thermal analysis, materials evaluation and selection, tolerance analysis and reverse engineering.
We frequently have clients coming to us to resolve specific technical challenges concerning product function. Uncertainty is our comfort zone, and we thrive on a challenge. As one of the UK’s most outstanding medical device developers, Neucin has the expertise to navigate these challenges.
Our mechanical engineers are passionate about thinking out of the box and finding solutions.
- Rapid prototyping of high fidelity ‘looks like’ ‘feels like’ prototype devices to accelerate the design and development process.
- Engineering analysis, empirical analysis, simulation, Finite Element Analysis (FEA), Tolerance analysis and modelling, CT scan data analysis and reverse engineering.
- CAD (Solidworks) detail design capability from simple components to complex assemblies including design for injection moulding from pilot low cavity to multi-cavity tooling.
- Bespoke assembly and test rig design, development and build to support test method validation and understand device performance.
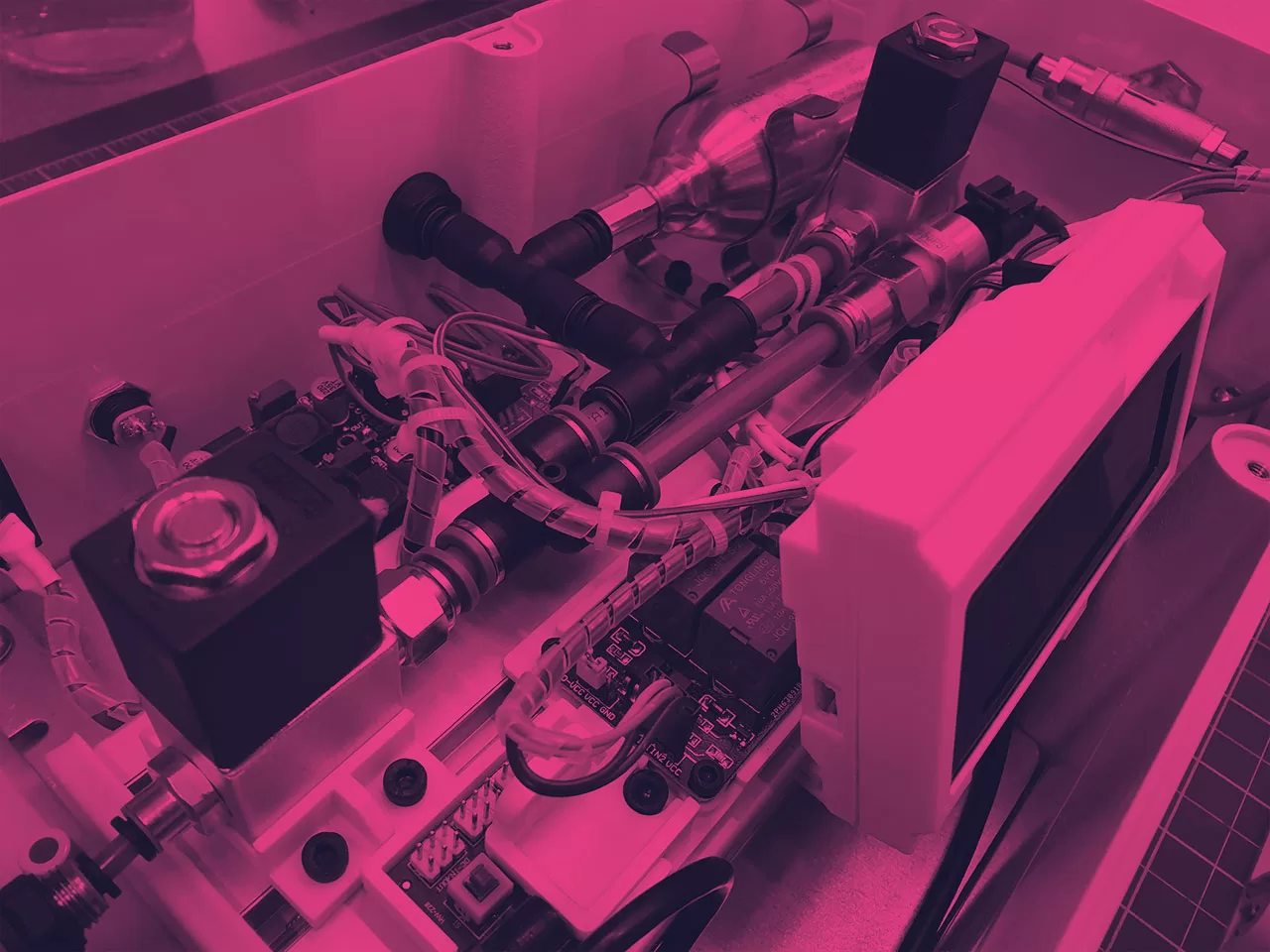
ELECTRONICS ENGINEERING
From one-off prototyping to fully embedded electrical engineering hardware design, Neucin excels in electronic design and engineering. Our clients often come to us seeking a “looks like”, “works like”, model or demonstrator, or the complete design for the manufactured product by the completion of the project.
Here at Neucin, we have the necessary expertise in the entire development cycle of embedded systems, including software and PCB design.
By working closely with our electrical engineering team, we can ensure the successful design of professional, functional products that meet the client’s unique needs and requirements.
- Electronics hardware design and product integration.
- Specification development, architecture definition, PCB design and bespoke sensor design and development.
- Software, programming and coding development including C, C++ and Python.
- Bespoke interfacing and HMI development.
- Design, development and build of proof of principle prototypes with integrated electronics and software for testing and early assessment.

TESTING & CHARACTERISATION
With Neucin’s in-house capabilities, performance testing, analysis, and evaluation are executed throughout the development process, from engineering studies to formal design verification testing.
Testing and characterisation are essential to the process of medical device product development.
- Device verification testing protocol development, execution and reporting (DVT).
- Design of experiments (DOE) and test method development.
- Bespoke test equipment design, build, verification and installation.
- Design verification testing planning, execution and reporting
- Device function and performance testing including mechanical compression, tensile and torque force testing and profiling.
- Development of custom testing jigs, fixtures and equipment for device testing and metrology.
- Inhaler testing capability for DDU, airflow resistance, pressure drop assessment, shot weight etc.

INDUSTRIALISATION
As a precision medical device product development company, we understand the intricacies of designing complex medical products. Experience tells us that we must consider manufacturing implications from the start of a new development to avoid costly project delays further down the line. We have developed specialised tools and techniques to ensure a seamless transition from the development phase into industrialisation and production scale-up.
These industrialisation-focused checks and balances include methods for analytical tolerance, templates for tracking and managing Design for Manufacture and Assembly (DFMA) actions, and tailored process risk assessment investigations and FMEAs.
- Development support from pilot-scale clinical volumes to CDMO design transfer and scale up.
- Design for manufacture (DFM) and design for assembly (DFA).
- Pre-clinical and clinical production assembly and test equipment design, installation and validation.
- Injection moulding, automated assembly and device testing design and validation support.
- Supplier selection and management.
- Instructions for use (IFU) and final packaging design and development.

REGULATORY COMPLIANCE
At Neucin, we value openness, respect, and trust. Being fully transparent, accredited and following the latest industry regulatory requirements is paramount to our reputation as a straight-talking and ground-breaking medical device company.
As a medical device design and development company, our obligation to all our clients is to ensure that their devices and products align with regulatory compliance. Throughout every department at Neucin, regulatory activities are in place and ongoing from the start of the process and remain 100% attached to the product throughout its entire lifecycle.
- Design and development process undertaken in accordance with ISO 13485 QMS requirements and ISO 14971 for device risk management.
- Design history file (DHF) and technical file creation and submission support.
- Regulatory standards review, compliance analysis and requirements capture.
- Risk management file creation and management including RMP, RMR, CTQ, RAC, Hazard identification analysis, dFMEA, uFMEA and pFMEA.